Aluminium oxide layers | ||||
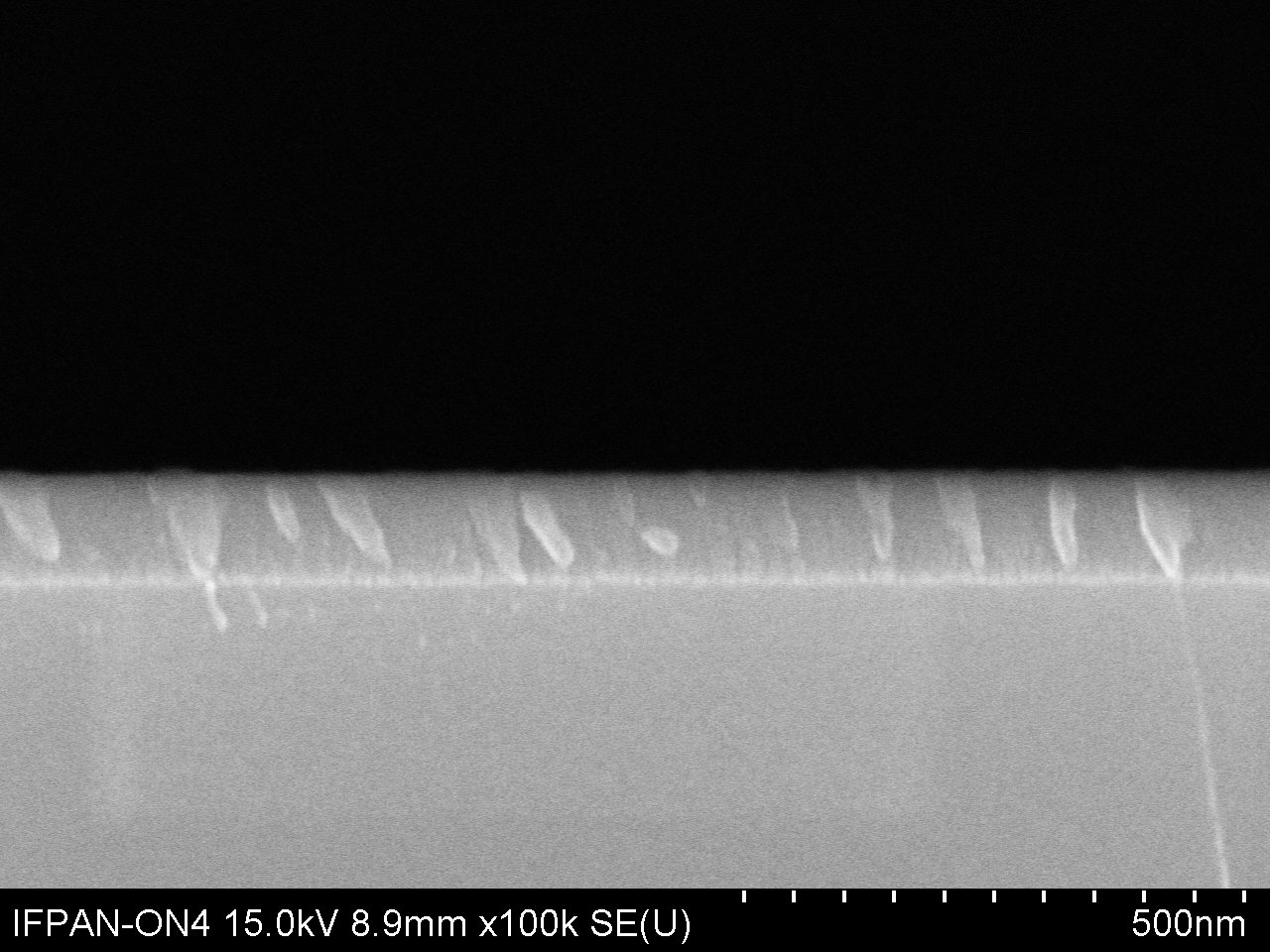
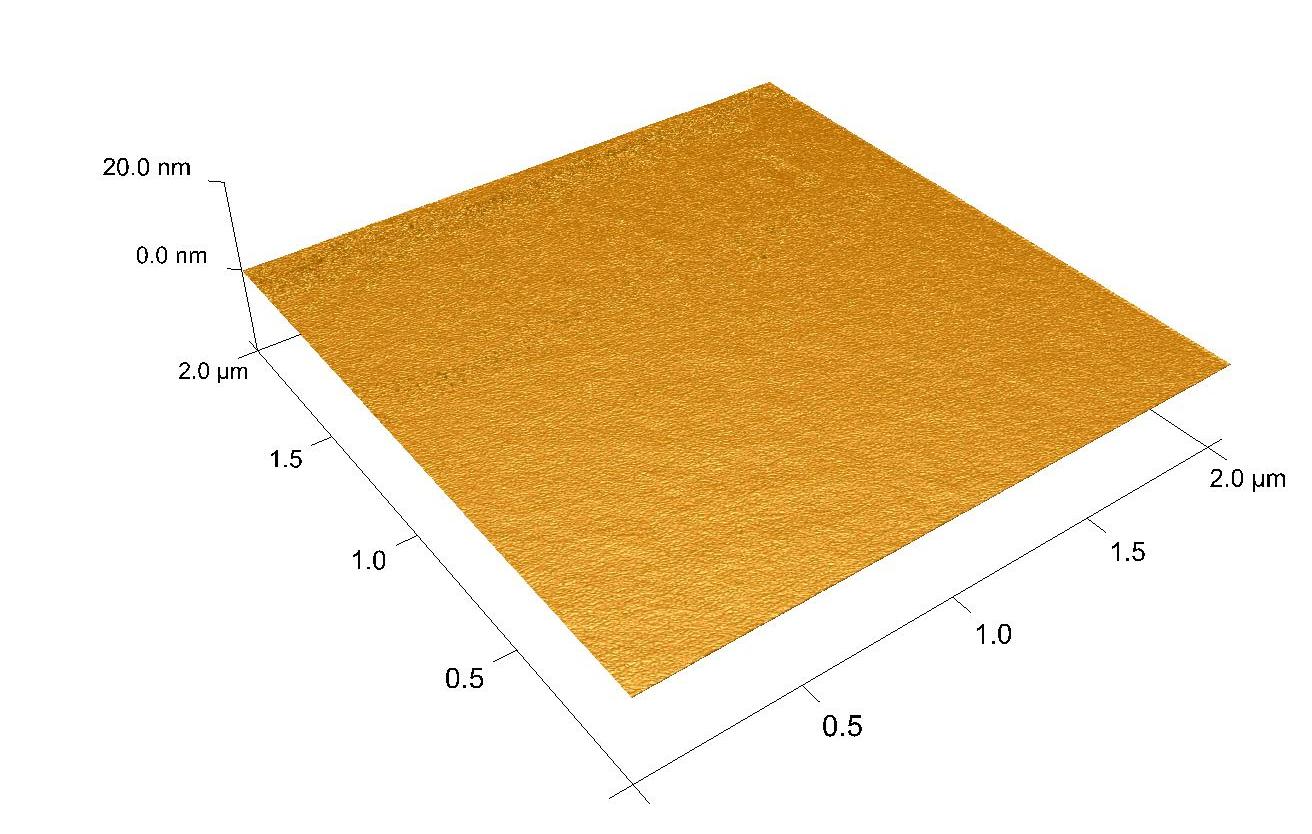
DescriptionAluminium oxide, also known as alumina (Al2O3), thin flims are obtained by atomic layer deposition (ALD) on range of substrates, like Si, GaN, SiC, graphene, SiO2 etc. The material is formed in a double exchange chemical reaction between two reactants (precursors), such as a deionized water (oxygen precursor) and a trimetylaluminum - TMA (aluminium precursor). Al2O3 can be grown at the temperature range from 25°C to 300°C. The maximum size of the substrate is 20 cm of a diameter.
Specification
ApplicationsAl2O3, due to its good optical, electrical and structural properties, can be used as an insulator in electronic devices, especially in "transparent electronics", as optical coatings with high refractive index in lasers and microscopes and as a protective or barrier layers of photovoltaic structures. In addition, this material can be used in medicine and dentistry for the manufacture of prostheses and implants. |
| |||